What is the order of stability of carbocation?
Carbocations are organic molecules that have a positively charged carbon atom. The stability of these molecules follows a certain order, which is known as the stability order of carbocations. The stability order can be described as follows:
1. tertiary > secondary > primary
2. allylic > benzylic
3. substituted > unsubstituted
4. bridgehead > non-bridgehead
A tertiary carbocation is the most stable type of carbocation, followed by secondary and primary carbocations. Allylic and benzylic carbocations are more stable than primary, secondary, and tertiary carbocations.
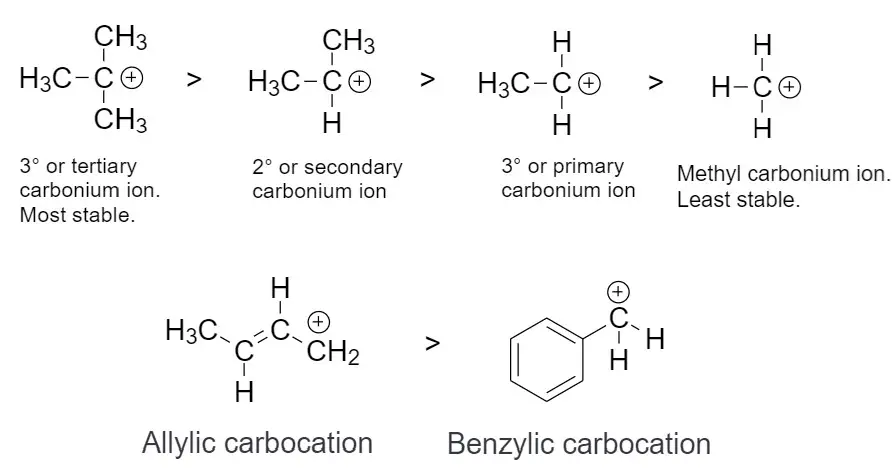
Substituted carbocations, such as alkyl or aryl-substituted carbocations, are also more stable than unsubstituted carbocations. Lastly, bridgehead carbocations are more stable than non-bridgehead carbocations due to the presence of electron-withdrawing substituents on the bridgehead carbon atom, which increases the stabilization of the carbocation by delocalizing the positive charge.
Why are tertiary carbocations more stable?
Tertiary carbocations are more stable than primary and secondary carbocations because they have more electron-donating groups attached to the positively charged carbon atom. These electron-donating groups stabilize the carbocation by delocalizing the positive charge, thus making the tertiary carbocation more stable than the primary and secondary carbocations. Additionally, tertiary carbocations have a smaller partial positive charge on the carbon atom than primary and secondary carbocations, which also contributes to their increased stability.
How do carbocations form?
Carbocations are formed when a carbon atom gains a positive charge. This can occur through a variety of mechanisms, such as the abstraction of a proton from an alkane by a strong acid, the addition of a Lewis acid to an alkene, or the reaction of a carbonyl compound with a strong base.