What is lanthanide contraction?
Lanthanide contraction is the phenomenon of the decrease in atomic radius of the elements of the lanthanide series as they move down the series. This decrease in radius is due to the increase in nuclear charge and the increased shielding effect of the inner electrons. This phenomenon is also known as the lanthanide effect.
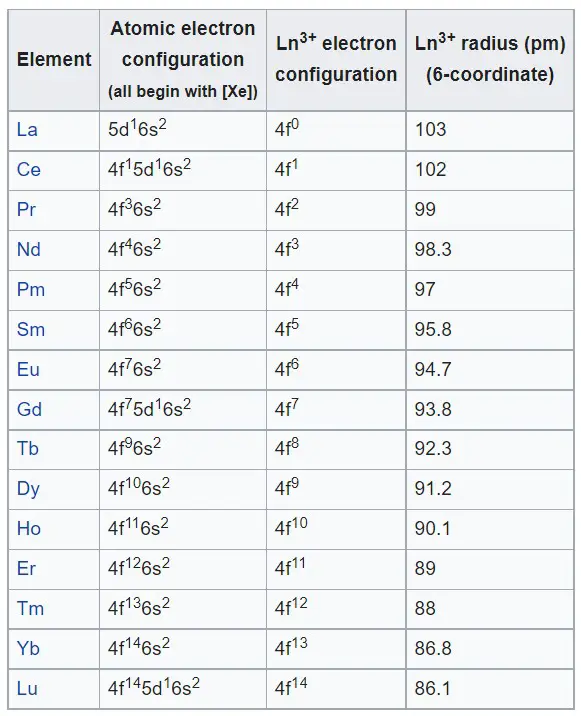
Consequences of lanthanide contraction
1. It affects the physical and chemical properties of the elements and their compounds.
2. It decreases the atomic radius of the elements in the lanthanide series as they move down the series.
3. It increases the ionic radii of the lanthanides, which affects their coordination chemistry.
4. It increases the electron affinity of the elements in the lanthanide series.
5. It affects the melting points of the elements in the lanthanide series.